Medical Food Status Visbiome.
Visbiome probiotic has a Medical Food status. Found in 1990’s Visbiome formulation has the largest amount of clinical studies and is the most studied probiotic formulation in the world. Therefore, Visbiome probiotic contains 8 species of freeze dried live bacteria and 10 times more potent than the average probiotic. As a high potency probiotic medical food, Visbiome manages the serious digestive disorders of IBS, ulcerative colitis and an ileal pouch and is intended to be used under medical supervision as per the FDA’s criteria.
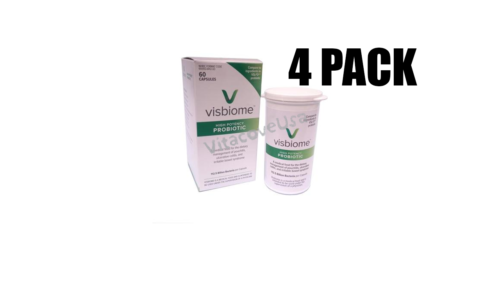
Visbiome probiotic is available in powder packets and capsule form. Each Visbiome capsule contain at least 112.5 Billion bacteria. While each Visbiome packet contain 450 Billion bacteria. The unique construction of new Visbiome capsules bottle allows it to be moister-resistant. Design with engineered desiccant sleeve to control moister absorption and provide the maximum potency preservation. Specially engineered bottle clicks, letting you know if closed properly. While specially design packets are made to prevent heat, light, moisture as well as contamination to maintain potency. Prescription strength of Visbiome available as well. Visbiome Extra Strength dispensed by prescription only.
Because Visbiome is refrigerated product, it is always shipped in a temperature controlled package.
What is Medical Food Status.
As defined in the Orphan Drug Act, medical food that are similar to prescription medications, is to be consumed under the supervision of a physician. However, patient are not required to obtain the prescription for medical food.